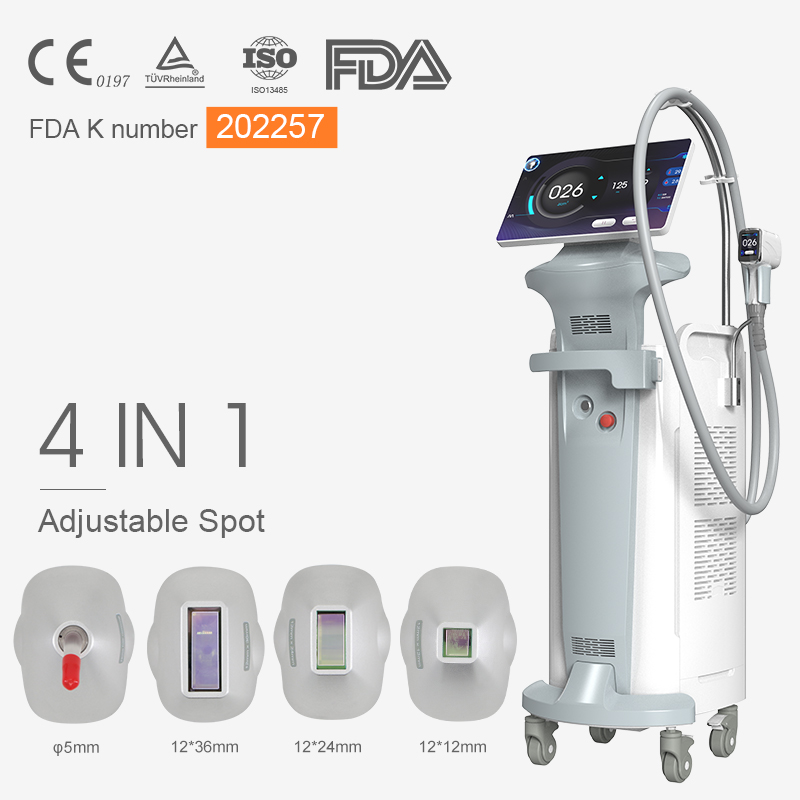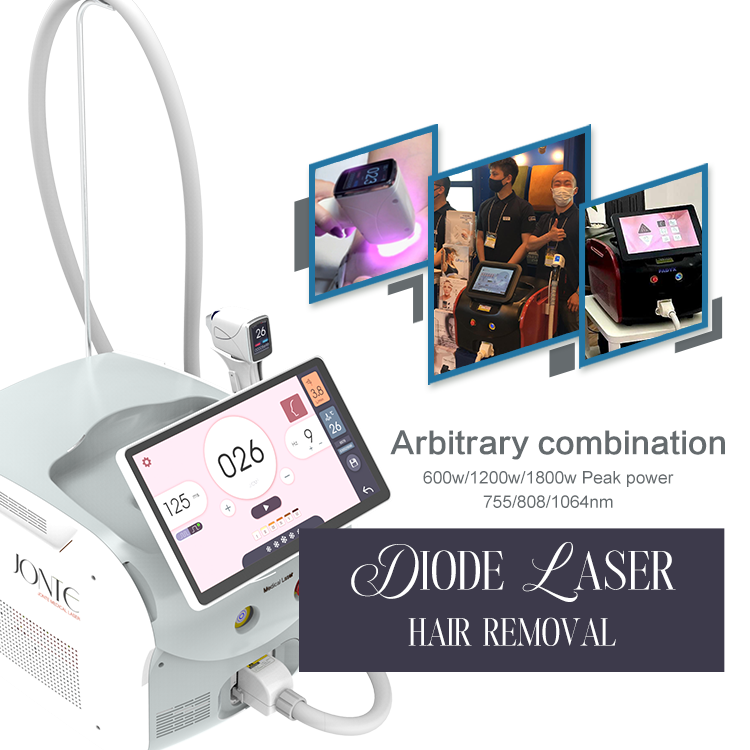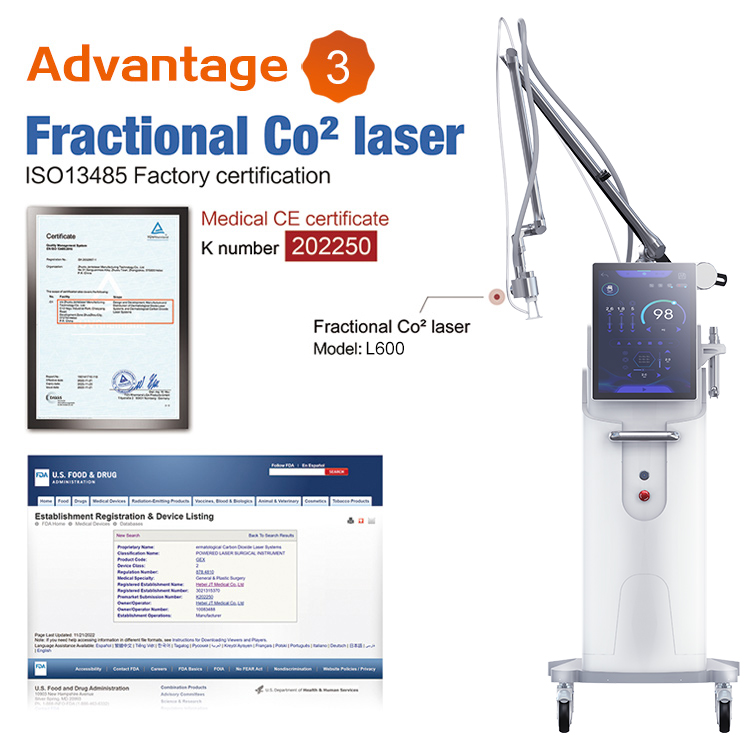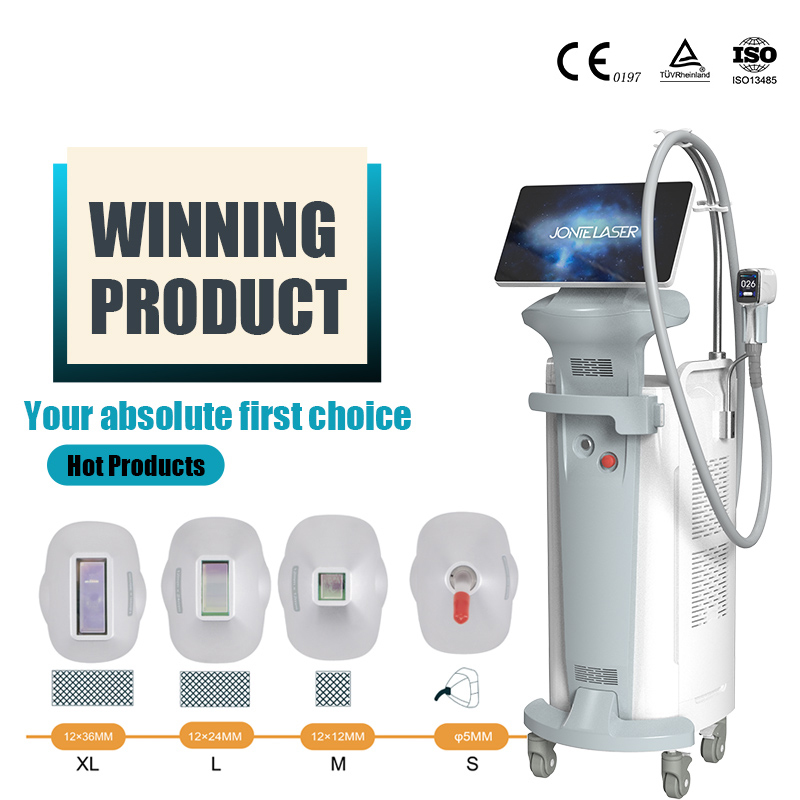
We are excited to announce that our advanced laser beauty devices, including the 808nm laser hair removal system and the CO2 fractional laser, have been awarded the esteemed FDA 510(k) Premarket Notification. This certification highlights our unwavering commitment to delivering innovative and high-quality beauty solutions to our customers.
About the FDA Certification
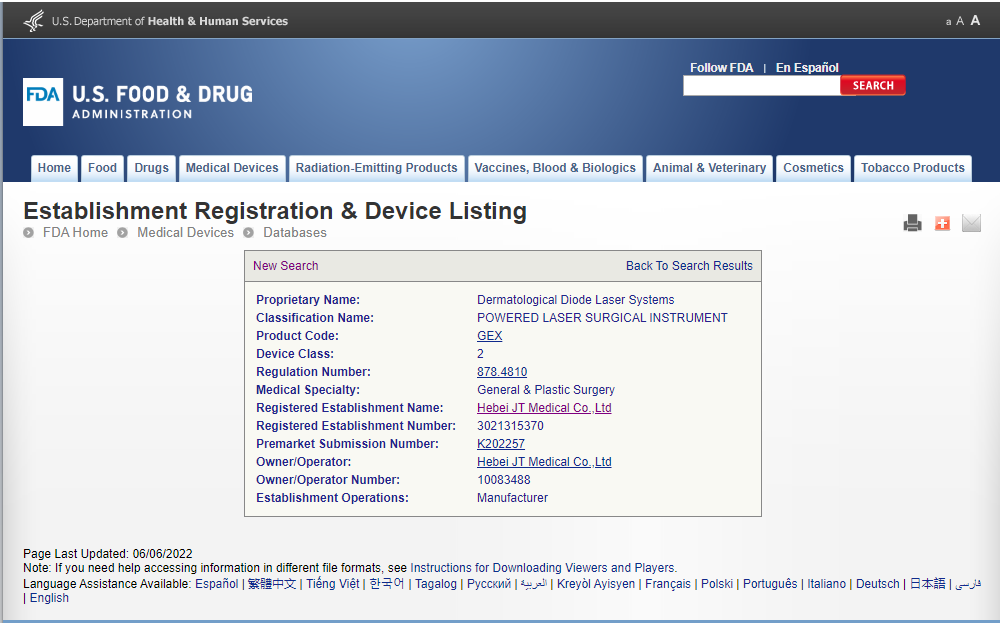
The FDA 510(k) clearance for our “Dermatological Diode Laser Systems” reaffirms the safety and effectiveness of our laser devices designed for dermatological and surgical applications. This certification ensures that our devices meet rigorous regulatory standards and are substantially equivalent to existing legally marketed devices.
Key Details from the FDA Notification:
Proprietary Name: Dermatological Diode Laser Systems
Classification Name: Powered Laser Surgical Instrument
Product Code: GEX
Device Class: 2
Regulation Number: 878.4810
Medical Specialty: General & Plastic Surgery
Registered Establishment Name: Hebei JT Medical Co., Ltd
Registered Establishment Number: 302135370
Premarket Submission Number: K202257
Owner/Operator: Hebei JT Medical Co., Ltd
Owner/Operator Number: 10083488
Establishment Operations: Manufacturer
Our Commitment to Quality and Innovation
For over 20 years, Jontelaser has been at the forefront of manufacturing and supplying top-tier beauty and medical systems. Our modern production facilities, equipped with 300,000-level clean production workshops, ensure that each device meets the highest standards of quality and safety.
With trademarks registered in 240 countries and our products exported to the Americas, Europe, Southeast Asia, the Middle East, and Africa, our international presence underscores our dedication to bringing advanced beauty solutions to a global audience.
About Our Laser Beauty Devices
Our FDA-certified laser beauty devices include:
808nm Laser Hair Removal System: Known for its precision and effectiveness in removing unwanted hair with minimal discomfort.
CO2 Fractional Laser: Utilized for skin resurfacing and treating various dermatological conditions, providing significant improvements in skin texture and appearance.
Join Us in Celebrating This Achievement
We invite our customers and partners to celebrate this significant milestone with us. The FDA certification of our laser beauty devices is a testament to our relentless pursuit of excellence and innovation in the beauty industry.
For more information about our products and this exciting development, please visit our website or contact us directly.
Contact Information:
Website: www.jontelaser.com
Email: [email protected]
Phone: 86-18617782197
Thank you for your continued support and trust in Jontelaser.